Are you analysing it carefully?
Some molecules may appear to incorporate one or more stereogenic centres, but close inspection can reveal an element of symmetry that is not immediately obvious (e.g. due to the complexity of the molecule or because of the way that it is drawn). The following examples reveal some of the subtle features that can be encountered. The conclusion is that one should be very careful when analysing structures, and the reliable way to find out if a structure is chiral is to build models of the structure and its mirror image, and then compare them atom-for-atom.
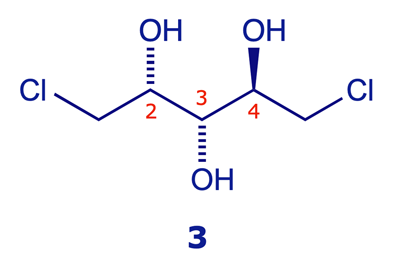
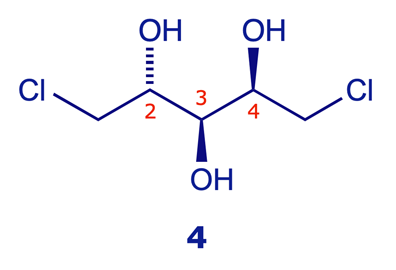
The amine 1 is chiral, the amine 2 is achiral (meso)
Both of the models below show the (R,R) stereoisomer of the chiral amine 1. Click the rotate C2 button on the second window and compare the result with the unrotated structure in the first – it's the same. This operation characterises a two-fold axis of symmetry. In contrast, the diastereoisomeric amine 2 is a meso compound: it has a plane of symmetry and is achiral (identical to its mirror image).
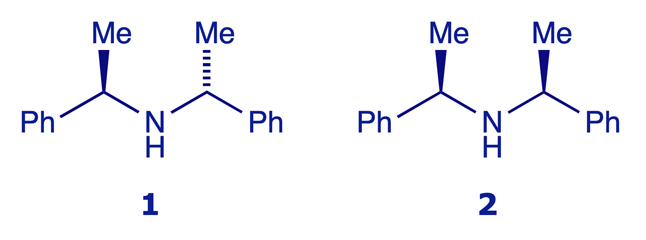
The triols 3 and 4 are identical – C(3) is not a stereogenic centre
The second pair of models show the triols 3 and 4, which at first sight look different (maybe diastereoisomers?) but are in fact the same structure. It makes no difference where the OH is placed on C(3): this carbon atom is not stereogenic because C(2) and C(4) are identical groups. Click the rotate y 180° button on the model labelled 4 and compare the result with the unrotated model 3 – they are the same.