2 Stereogenic centres
2.1 Configurational isomerism and chirality
Configurational isomers differ in the spatial arrangement of their atoms and can only be interconverted by breaking bonds (and not by free rotation about single bonds). In the case of carbon compounds the isomers do not interconvert at room temperature and can be separated. Configurational isomers fall into two categories: enantiomers and diastereoisomers.
Example:
2.1.1 Chiral centres (stereogenic centres)
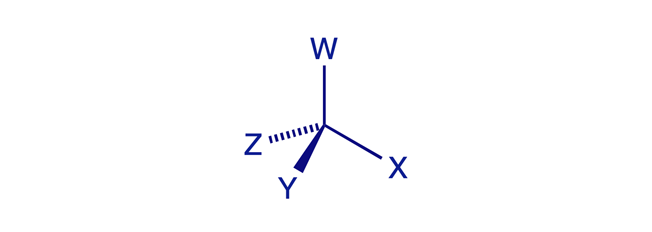
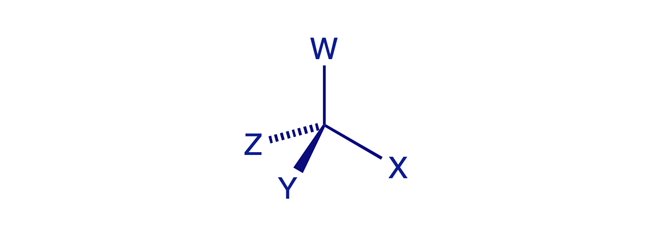
Molecular chirality ('handedness') is usually, but not always, caused by the presence of one or more chiral (stereogenic) centres in the molecule. A carbon atom is a chiral (stereogenic) centre when it is tetrahedral (sp3-hybridised) and has four different groups (ligands) attached to it. The word chirality originates from the Greek word for 'hand' — chirality is handedness.
A simple way to determine whether an object is chiral is to look at it in a mirror and ask: Is the mirror image different from the original? Any tetrahedral structure will be 'handed' unless at least two of its four vertex corners are identical. The sp3 carbon atom in CWXYZ meets this condition and is therefore stereogenic.
In the general case, any object will exhibit handedness if it has no plane of symmetry. Many everyday items are chiral, but we rarely focus on it. Look around and see if you can identify this property in nearby objects.
2.1.2 Enantiomers
Enantiomers are stereoisomers which are non-superimposable mirror images of one another. They have identical physical properties (solubility, m.p., b.p., NMR, IR spectra etc.).
Examples:
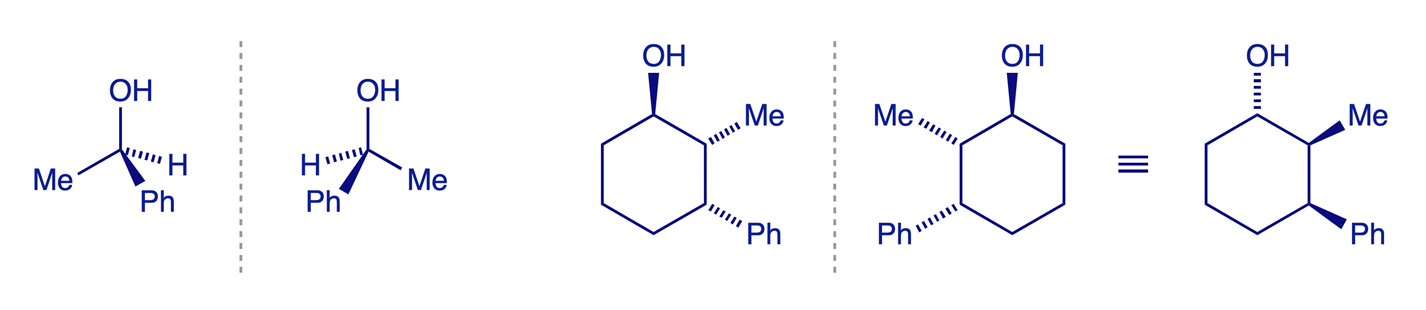
Chirality is a property of the molecule as a whole. Remember: A molecule is chiral if it is not superimposable on its mirror image. ONLY isomers that are non-superimposable mirror images are enantiomers of each other.
2.1.3 Diastereoisomers
This section uses Fischer projections, which are revised below in Section 2.2.2.

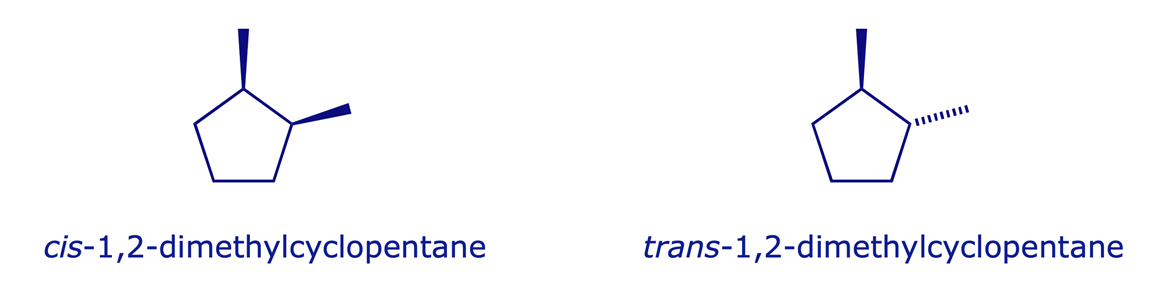
Diastereoisomers can be defined as stereoisomers which are not enantiomers. In common with enantiomers, diastereoisomers have the same constitution but different configurations and are non-superimposable, but they differ from enantiomers in one crucial respect: they are not mirror images. Isomerism of this type is possible when a molecule has more than one stereogenic centre. A simple example is the 2,3-dichloropentane molecule shown below, in which C(2) and C(3) are stereogenic carbons.
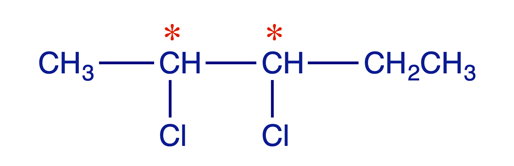
The molecule has two stereogenic centres, and four stereoisomers are possible. The relationships between the four stereoisomers are indicated.
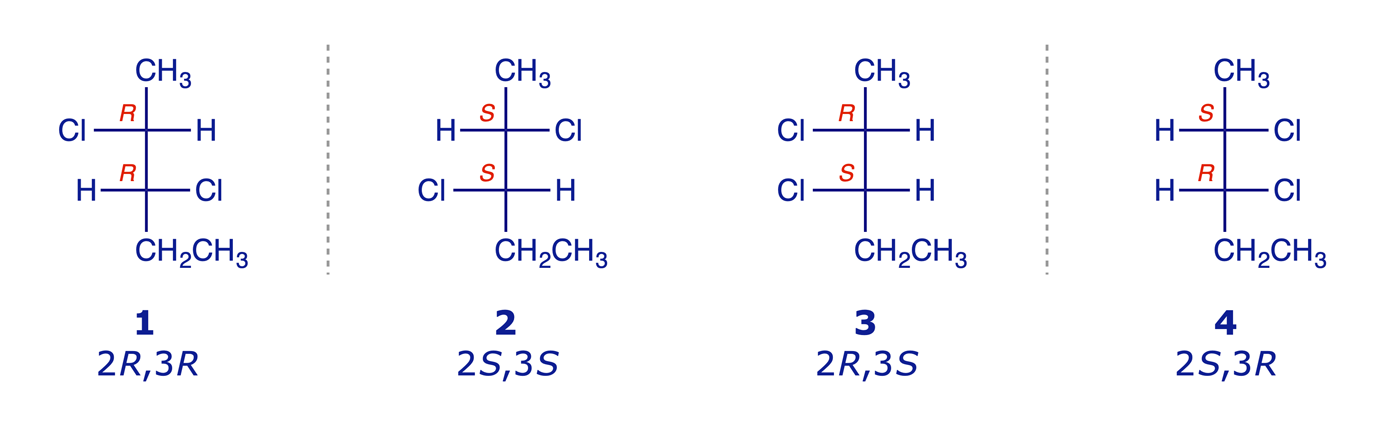
- 1 and 2 are enantiomers
- 3 and 4 are enantiomers
- 1 and 3 (or 4) are diastereoisomers
- 2 and 3 (or 4) are diastereoisomers
Diastereoisomers are, essentially, different compounds. They have different physical properties (solubility, m.p., b.p., NMR, IR spectra etc.) and can be separated by physical methods such as fractional distillation. Distillation of 2,3-dichloropentane gives two fractions, one containing equal proportions of 1 and 2, and the other containing equal proportions of 3 and 4 (the enantiomers are not separable by distillation). Geometrical isomers also satisfy the definition for diastereoisomers.
Examples:
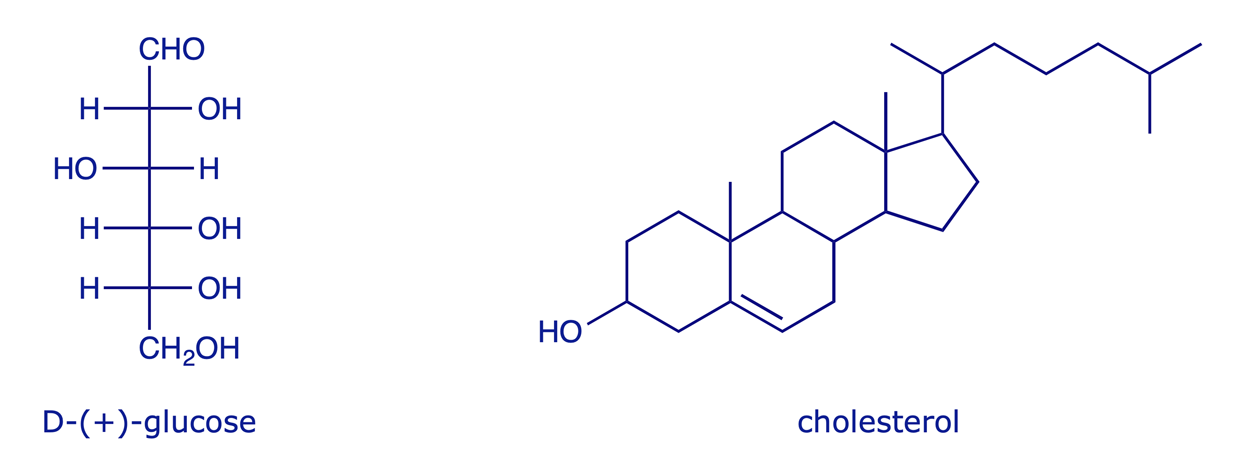
Compounds with more than two stereogenic centres are common in nature. In general, for a molecule with n stereogenic centres, 2n stereoisomers are possible. Glucose has four, and so is one of 16 possible stereoisomers. Cholesterol, with eight, has 28 (256) possible stereoisomers, of which only one occurs in nature. Click on the image to highlight the eight stereogenic centres.
Although these large numbers might be disconcerting, in principle stereochemisty is very simple to understand. A chiral centre can only be either R or S and there is no in-between. But there are subtle points which can lead to difficulties. We will therefore progress from very basic principles to a quite detailed look at stereochemistry. First of all we will take a closer look at enantiomers and diastereoisomers, then study how they can be distinguished and separated. Finally we will look at their synthesis.
2.1.4 Meso compounds
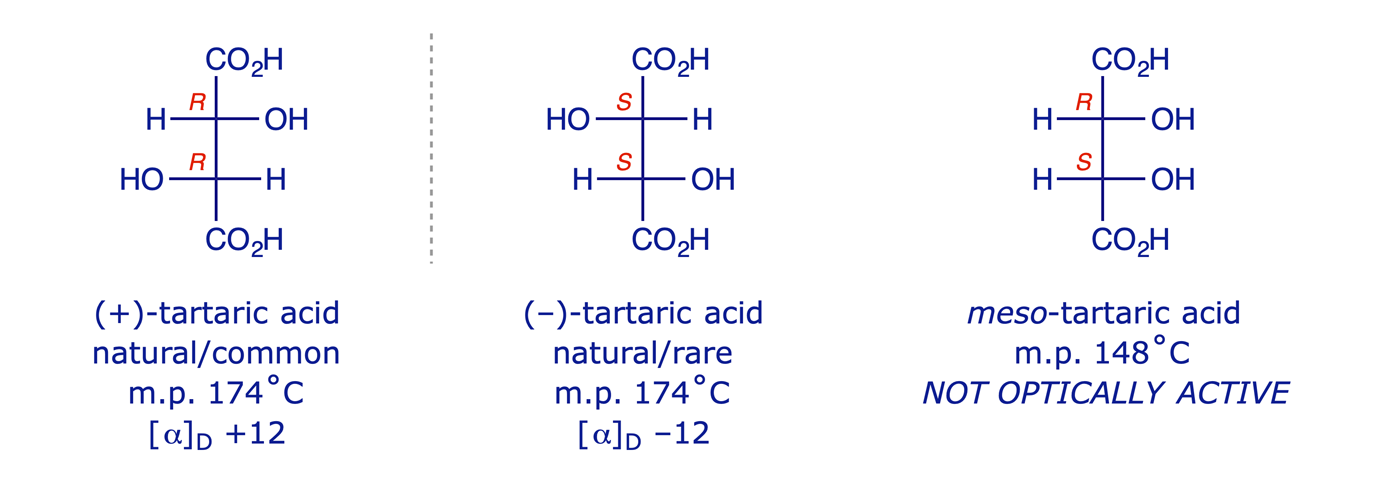
Some molecules have stereogenic centres but also have a plane or centre of symmetry which causes the molecule as a whole to be achiral. Earlier we looked at 2,3-dichloropentane and identified four stereoisomers (two pairs of enantiomers). Now consider 2,3-dichlorobutane. We can draw the four structures (22) that we might expect.
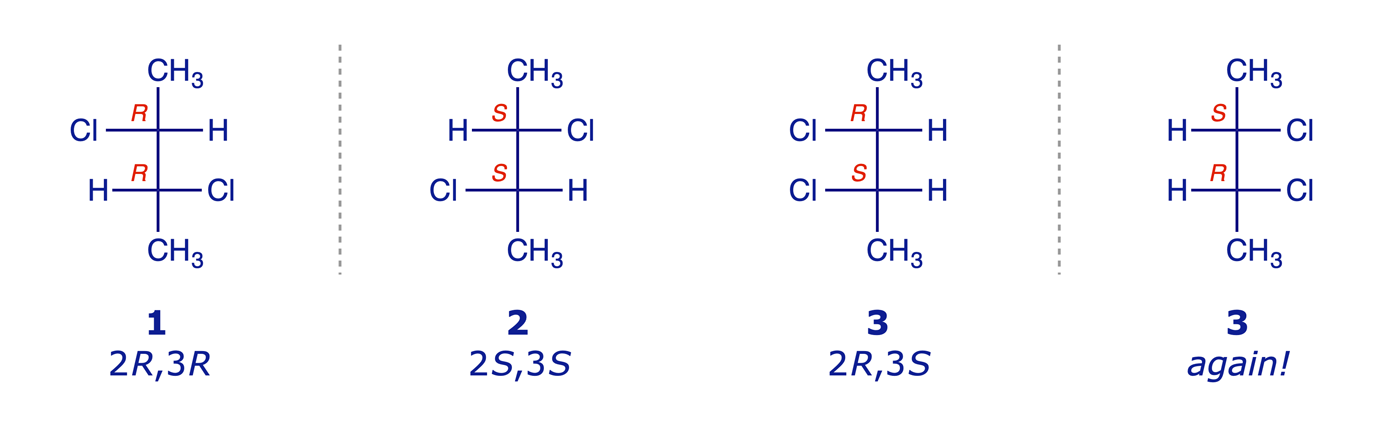
But molecule 3 has a plane of symmetry, and so it is achiral and not optically active. It is a meso compound (one whose molecule is superimposable on its mirror image even though it contains stereogenic centres). The optical activity due to one stereogenic centre is cancelled by that of the other, which is its mirror image.
Perhaps the best known example of this is tartaric acid, which exists as three stereoisomers (two enantiomers and a meso compound). Crystals of potassium hydrogen (R,R)-tartrate are often to be found in the bottom of wine bottles.
2.2 Identifying stereoisomers
In addition to the CIP system, in which a compound is classified by the assignment of R or S to its stereogenic centre(s), there are other classes of stereoisomer based on different structural comparisons. As with the CIP system, it is necessary to analyse a structure very carefully in order to classify it.
2.2.1 Using R and S descriptors
The most important system for defining chirality at individual chiral centres utilises the Cahn-Ingold-Prelog (CIP) sequence rules. Each stereogenic atom is assigned a descriptor R or S. For revision of this method, see section 2.2.5 below.
2.2.2 Using D and L descriptors
Although the universality of the R/S nomenclature system is clear, there is widespread use of an older system in which the absolute configuration of some natural molecules is specified according to a convention developed for the carbohydrates, and used by Fischer. In this method the absolute configuration of a molecule is defined as D or L according to its relationship with one or other enantiomers of the simplest carbohydrate molecule, glyceraldehyde.
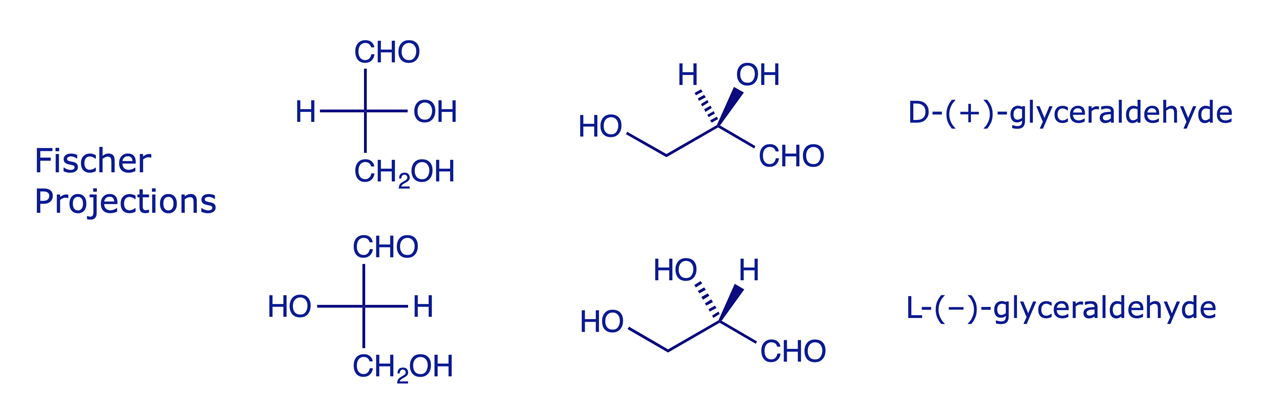
The glyceraldehyde molecules were originally defined as D and L on the basis of their optical activity (one is Dextrorotatory and the other is Laevorotatory), some fifty years before their absolute configurations were actually confirmed. Other carbohydrates and amino acids were assigned by comparison to them.
Thus, a sugar, represented in a Fischer projection with the most oxidised carbon at the top, is defined as:
- from the D-series if the bottom secondary OH is on the right
- from the L-series if the bottom secondary OH is on the left
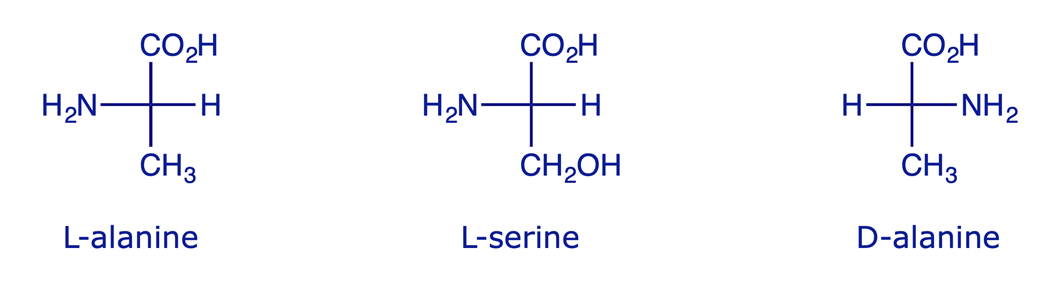
Similarly, L-amino acids have the amine group on the left hand side when drawn in a Fischer projection.
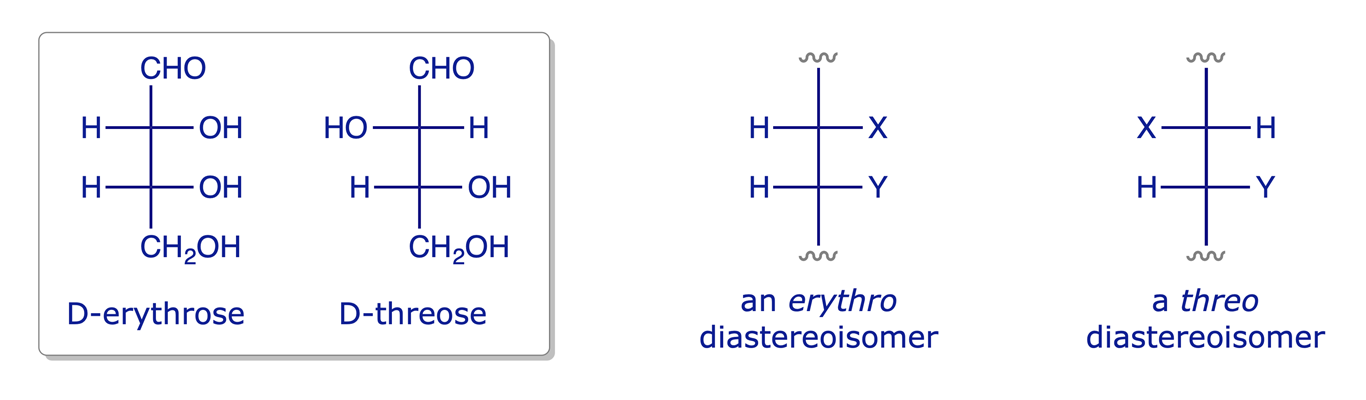
2.2.3 Relative configuration: erythro and threo nomenclature
Erythro and threo are sometimes used to describe the relative configuration of two stereogenic centres in a pair of diastereoisomers. The words have a carbohydrate origin, and relate to Fischer projections, thus:
- When the carbon chain is vertical and like substituents are on the same side of the Fischer projection, the molecule is referred to as an erythro diastereoisomer.
- When the carbon chain is vertical and like substituents are on opposite sides of the Fischer projection, the molecule is a threo diastereoisomer.
The 'like' substituents do not have to be exactly the same, and the stereogenic centres do not have to be adjacent, so these terms can seem rather loose. They are only useful in special contexts such as the analysis of stereoselective synthetic reactions.
2.2.4 Relationship between different conventions
To summarise, there are various ways in which the stereochemical features of a molecule can be defined:
- Using (R/S) nomenclature [Cahn-Ingold-Prelog] — this system is universal
- Using (D/L) nomenclature [Fischer] — this system is anachronistic but still widely used
- According to the sign of optical rotation, using the prefix (+)- or d- for dextrorotatory compounds (positive sign of rotation); and (−)- or l- for laevorotatory compounds (negative sign of rotation). Either (±)- or d,l- are used to indicate racemic mixtures.
IMPORTANT POINTS TO REMEMBER:
- There is no general relationship between these three conventions
- A compound should be described using more than one prefix, e.g. (S)-(+)-alanine, in order to define it fully
- A sign of rotation in the polarimeter gives no clue as to the configuration of a chiral molecule — the descriptors (e.g. R or S) are simply to help us when we wish to refer to one particular structure.
It is important to understand the different methods for 'drawing' stereochemistry. Building and inspecting molecular models is the best way to develop an appreciation of what structures drawn on paper actually mean.
2.2.5 R and S Descriptors: The Cahn-Ingold-Prelog (CIP) sequence rules
R. S. Cahn, C.K. Ingold and V. Prelog, Experientia, 1956, 12, 81.
R. S. Cahn, Journal of Chemical Education, 1964, 41, 116.
A carbon atom is a chiral (stereogenic) centre if it is tetrahedral (sp3) and has four different groups (ligands) attached to it.
We need ways to define chirality at individual chiral centres, and of molecules as a whole. The systems used have been developed over many years and can be confusing. The most important system utilises the Cahn-Ingold-Prelog (CIP) sequence rules.
Procedure
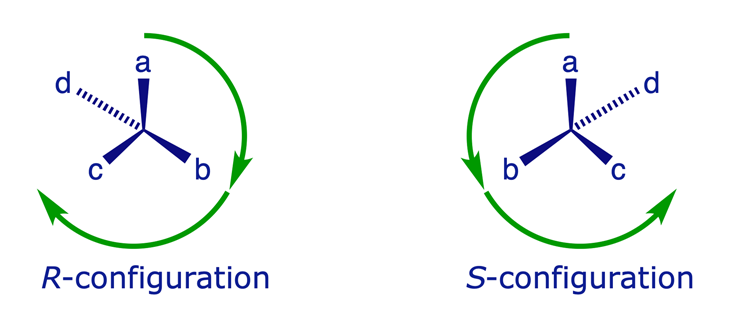
- Identify the four different groups attached to the chiral atom. Assign to each a priority a, b, c, or d, using the Sequence Rules, such that a > b > c > d.
- Orient the molecule in space so that one may look along the bond from the chiral atom to the group with the lowest priority, d. Trace a path from a to b to c. If the path describes a clockwise motion, the configuration of the atom is R. If the path describes an anticlockwise motion, the configuration of the atom is S.
Sequence Rules
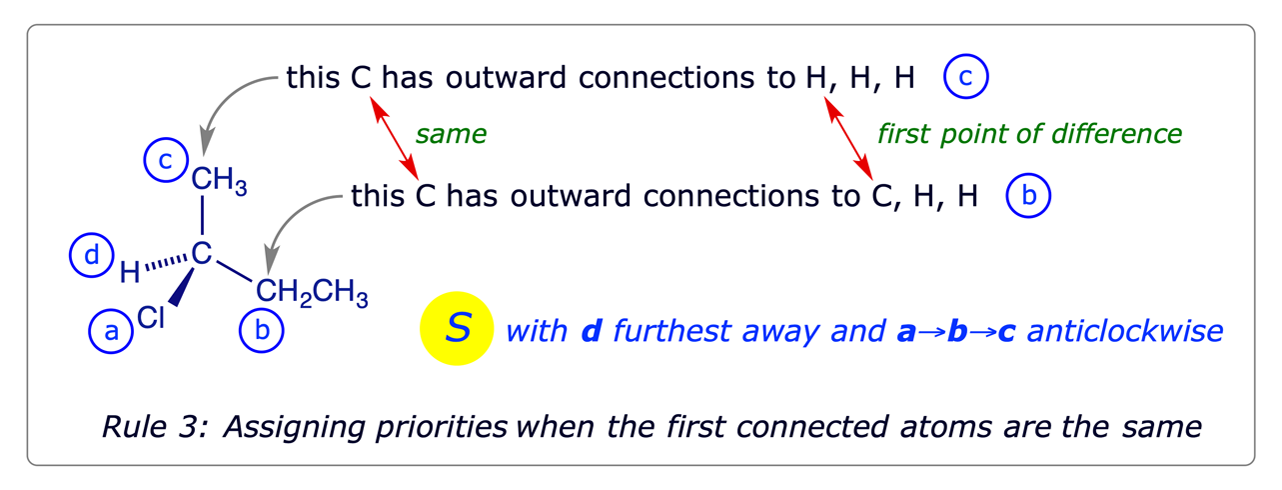
- For the atoms attached directly to the chiral atom, higher atomic number precedes lower.
- For isotopes, higher atomic mass precedes lower.
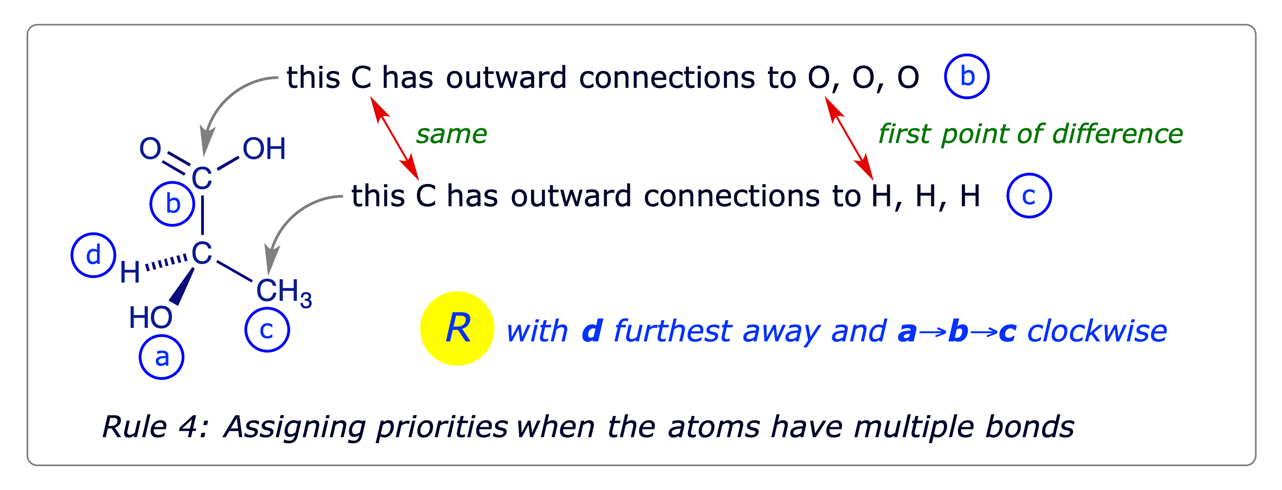
- When two atoms directly attached to the chiral atom are the same, work outward concurrently along the two chains atom by atom until a point of difference is reached. Assign the priorities at that first point of difference, using rules 1 and 2. For branched chains, proceed along the branch of higher priority first.
- Double and triple bonds are treated by assuming that each such bonded atom is duplicated or triplicated respectively. Some examples of this are shown below.

Illustrative examples:

2.2.6 Specification of alkene geometry using E and Z descriptors
The Cahn-Ingold-Prelog sequence rules are also used to name tri- and tetra-substituted alkenes, for which the cis/trans terminology is not useful. Instead the terms E and Z are applied, as follows:
- For each end of the double bond, prioritise the two attached groups as a and b using the Sequence Rules.
- Describe the relative locations of the a groups using the E or Z descriptor as follows: E = apart (German: entgegen); Z = together (German: zusammen).

