Chiral reagents
Mosher's enantioselective reducing agent
In the reduction of acetophenone with sodium borohydride, the achiral reagent can approach either face of the planar carbonyl group with equal facility. The alternative transition state structures are enantiomeric and the reaction rates the same, so the product is obtained in racemic form, i.e. as a 50:50 mixture of (R)-1-phenylethanol and (S)-1-phenylethanol.
While seeking reagents that would reduce carbonyl groups enantioselectively, Yamaguchi and Mosher found that partial decomposition of lithium aluminium hydride (LiAlH4) with a chiral alcohol gave a modified chiral reducing agent capable of reducing acetophenone preferentially from one of its carbonyl faces. The method is simple, and an early example of the use of a chiral auxiliary to convert an achiral reagent into an enantioselective variant. It should be noted that the level of selectivity achieved (e.e. 68%) is not ideal in a practical sense because of the difficulties associated with purifying (i.e. resolving) the product in order to obtain a single enantiomer.
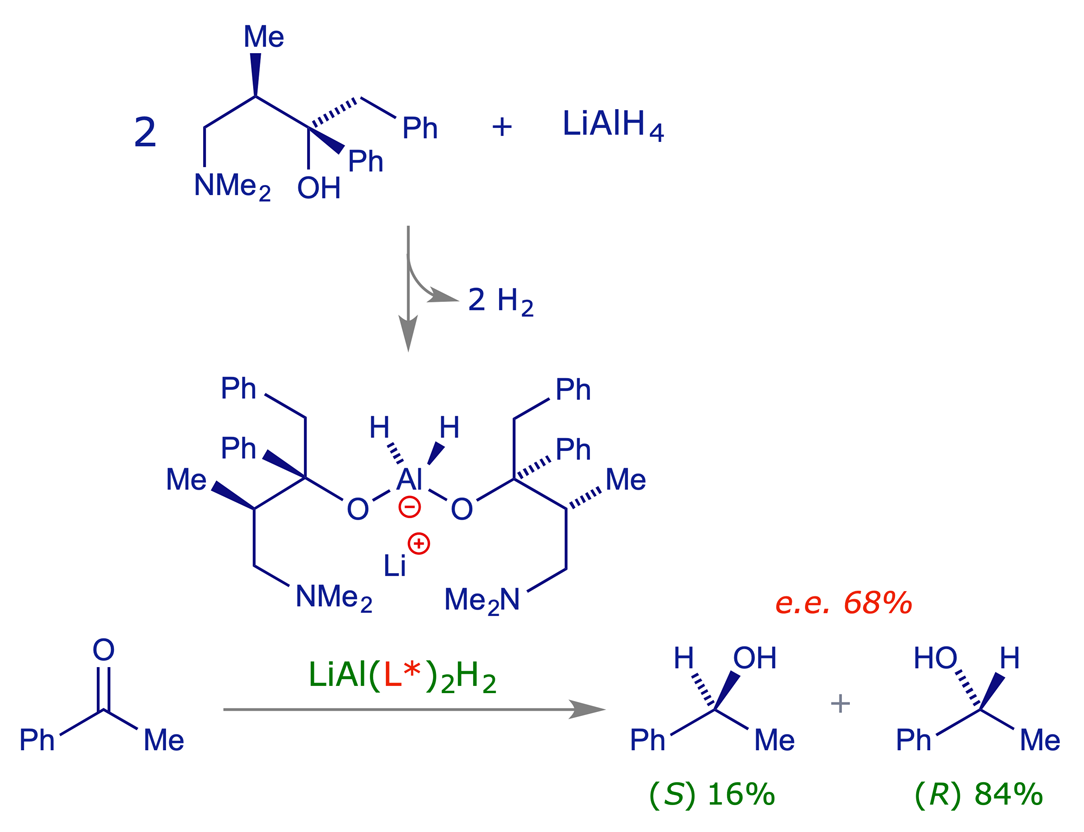
Asymmetric reductions with chiral reagents from lithium aluminum hydride and (+)-(2S,3R)-4-dimethylamino-3-methyl-1,2-diphenyl-2-butanol; S. Yamaguchi and H. S. Mosher, J. Org. Chem., 1973, 38, 1870–1877 (DOI: 10.1021/jo00950a020).
