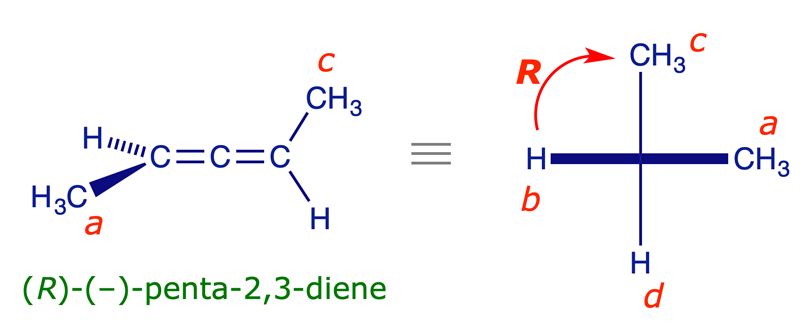
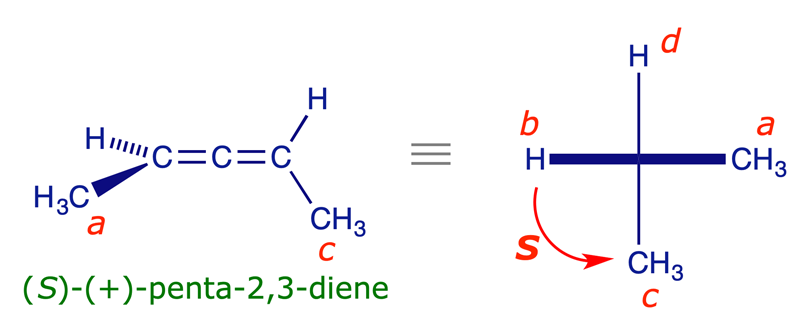
Applying (R,S) descriptors to allenes
1,3-Disubstituted allenes have fixed perpendicular dissymmetric planes, another type of structure that is naturally 'handed.' If the two groups in one of the planes (i.e. at one end) of the allene are the same, then the other plane of the allene will be a plane of symmetry and the molecule will be the same as its mirror image. However, if neither of the two planes of an allene is a plane of symmetry (i.e. neither end has two identical groups), then the mirror images are non-superimposable and the structure is chiral. One of the simplest examples is 2,3-pentadiene, which is shown in the interactive model below.
The procedure for assigning (R,S) stereochemical descriptors to allenes is similar to that for biaryls. The molecule is viewed along its axis and the relevant substituents are projected on to a plane at right angles to the axis. The groups are prioritised using the Sequence Rules with the extra proximity rule, which requires that near groups precede far groups.